
Exclusive: GeoVax’s CEO David A. Dodd in an Engaging Conversation with PharmaShots
Shots:
-
In an extended cut to our Spotlight Interview, PharmaShots presents an exclusive interview session with David A. Dodd, Chairman, President, and CEO at GeoVax labs
-
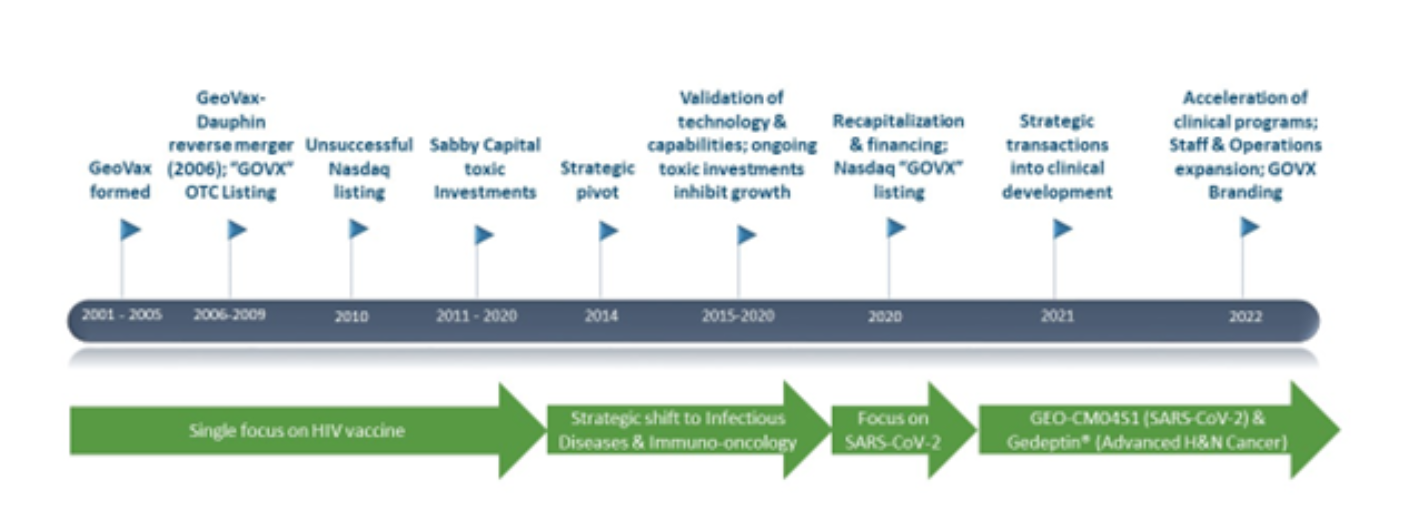
David speaks about the company’s rich history and highlights the journey through the years and the strategic shift from being a single-focus HIV vaccine developing company to an infectious and Immuno-oncology company
-
David discusses in great depth GeoVax’s technology platforms viz. Modified Vaccinia Ankara and Gene-Directed Enzyme Prodrug Therapy
Himanshu: Shall we start with a quick introduction about GeoVax? (The history, the technology, vision, mission, founder etc.)
David: GeoVax Labs, Inc. is a clinical-stage biotechnology company developing vaccines and immunotherapies against infectious diseases and cancers using novel proprietary platforms.
Vision: Our vision is to be a sustainable, profitable growth-oriented developer of therapies and vaccines against cancer and infectious diseases, recognized as a key contributor to improving options available to patients worldwide within our areas of focus. We also envision GeoVax as a preferred career-development organization to which highly innovative, competitive individuals aspire to develop careers.
Mission: Our mission is to improve lives worldwide by providing vaccines and therapies preventing or treating some of the world's most challenging cancers and infectious diseases, improving shareholder/stakeholder value and providing motivating career opportunities for our team.
Himanshu: We could see there are two technologies listed on the GeoVax’s website MVA-VLP Technology & Gedeptin Technology. So, what are these technologies, who developed them? How they work and what are their applications?
David: GeoVax utilizes two robust core technology platforms:
-
Modified Vaccinia Ankara (MVA) for creating vaccines for the prevention of various infectious diseases, and the basis for creating immuno-cancer therapies.
-
Gene-Directed Enzyme Prodrug Therapy (GDEPT) for the treatment of solid tumors with an initial focus on advanced head and neck cancer.
GeoVax’s vaccines are constructed to induce broader immunity through inclusion of multiple antigens into a single virus/vaccine platform. This is possible through the use of the company’s Modified Vaccinia Ankara (MVA) vaccine platform, a large virus capable of incorporating multiple antigens into a vaccine platform.
Utilizing MVA, as a vaccine vector, allows for the targeting of multiple sites on a pathogen or cancer cell. Doing this is intended to result in a more robust and durable protective immune respons. In addition, using MVA as a vaccine platform allows for the construction of vaccines which are capable of generating virus-like particles (VLPs) in the person receiving the vaccine.
The production of VLPs in the person being vaccinated is intended to mimic viral production that occurs in a natural infection, stimulating both the humoral (antibody) and cellular (T-cell) arms of the immune system to recognize, prevent, and control future infections.
MVA vectored vaccines can elicit durable (long-acting) immune responses while also possessing an excellent safety profile. MVA-VLP vaccines are designed to mimic authentic viruses in form but are not infectious or capable of replicating. As a result, VLPs can cause the body’s immune system to recognize and kill targeted infectious agents to prevent an infection or can be designed to target cancerous cells resulting in inhibited growth or destruction of tumors. VLPs can also train the immune system to recognize and kill virus-infected cells to control infection and reduce the length and severity of disease.
We design our MVA-VLP vaccines such that, when VLPs for viruses like Ebola or Marburg are produced in vivo (in the cells of the recipient), they present the viral protein antigens to the host cells in a manner similar to those of a natural infection.
MVA, as a vaccine, was developed in the 1950s/1960s as a safer smallpox vaccine for use in immune-compromised individuals. The safety and utility of MVA as a vaccine and as a vaccine-platform is well documented and recognized by public health authorities worldwide.
GeoVax obtained rights from the U.S. NIH to develop MVA as a vaccine for smallpox and Mpox (GEO-MVA), as well as also having rights from the NIH to use MVA as a vaccine vector.
GeoVax also holds exclusive rights to use the City of Hope Medical Center's synthetic MVA (sMVA) platform for the manufacture and distribution of Covid-19 vaccines including GEO-CM04S1, our multi-antigenic Covid-19 vaccine currently in multiple Phase 2 studies.
GeoVax is transitioning to an advanced, MVA manufacturing process with the goal of significantly increasing the manufacturing yield and volume capacity in response to potential epidemic and pandemic needs. The advanced MVA manufacturing utilizes a patented, proprietary continuous avian cell line system (AGE.1) for manufacturing MVA (modified vaccinia Ankara)-based vaccines and immunotherapies.
Currently, MVA vaccines are manufactured in cells cultured from chicken embryonic fibroblasts (CEF), a suboptimal and time-consuming process useful primarily for niche markets and stockpile reserves. Transitioning to the AGE.1-based system is anticipated provide lower-cost, scalable versatility for broad MVA vaccine and immunotherapy applications. In addition, the advanced MVA manufacturing process is intended to expand MVA-based vaccine production from stockpile-based solutions for niche medical markets to include the ability respond to world needs on a timely basis, whenever and wherever they arise.
We believe that GeoVax will be the first supplier of MVA-based vaccines to implement such a transformative manufacturing process and intends to become the first U.S.-based supplier of the MVA vaccine to prevent Monkeypox, Smallpox and other pox-related viruses. The initial candidates for the advanced manufacturing process include GEO-MVA (addressing Monkeypox/Smallpox) and GEO-CM04S1 (Covid-19 vaccine).
The mechanism of action for Gedeptin is applicable to a broad range of needle-accessible tumors. While GeoVax is initially focusing its efforts on accelerating the development of Gedeptin for advanced head and neck squamous cell carcinoma (HNSCC). Following success of this unique therapy in HNSCC, other tumor types will be addressed in a similar fashion. Such tumors include soft tissue sarcoma, glioma, and cancers of the cervix, breast, prostate among others, which represent a significant and unmet medical need.
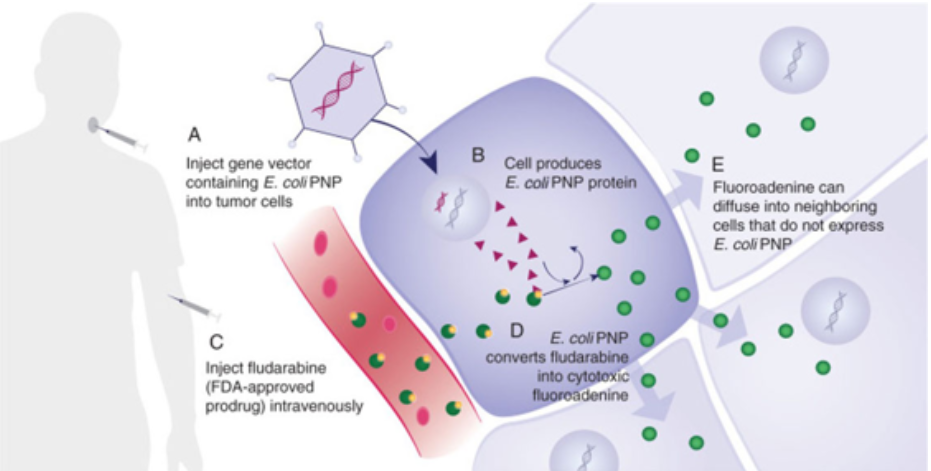
Gedeptin® represents “Gene Directed Enzyme Prodrug Therapy” or GDEPT, which is a platform technology designed to reduce or eliminate targeted, tumors. The mechanisim of action (MOA) is explained in the schematic above, following five steps:
- Inject a gene vector containing E. coli PNP into the targeted tumor cells
- The cell produces E. coli PNP protein
- Infuse fludarabine, an FDA-approved hematological chemotherapeutic agent, intravenously
- E. coli PNP converts fludarabine into a highly-cytotoxic fluoroadenine; if fludarabine encounters a tumor untreated with the gene vector, nothing occurs
- Fluoroadenine can diffuse into neighboring cells that do not express E. Coli PNP, however,
The result is an unprecedented level of cell-killing activity via a novel tumor killing mechanism. This facilitates the destruction of refractory solid tumors and, is “tumor agnostic”. The initial targeted indication is against advanced head and neck cancer. Additional monotherapy against other solid tumors is planned, as well as clinical development of combination therapy, using Gedeptin in conjunction with immune checkpoint inhibitors, for which ongoing preclinical studies are highly encouraging.
Himanshu: This is so amazing. Thanks for sharing the information with us. We could see that your lead molecule is a COVID next generation vaccine. Why do we need a next-gen vaccine for COVID-19?
David: Next-generation COVID-19 vaccines are needed because the current authorized vaccines are recognized as not sufficiently robust (meaning, breadth of immune protection) nor durable (meaning, their immune response appears to only last approximately 2-4 months). In addition, the current vaccines have to be reconfigured every time there’s a new significant variant. First it was Delta, then the Omicron XBB1.5, then we started talking about subvariant EG.5 (Eris) and now, we’re addressing JN.1. Such variants will continue to emerge. Unfortunately, by the time a vaccine reconfiguration against a new variant is completed, , we’re halfway to another emerging variant of concern.
There has been a high disappointment with the current authorized Covid-19 vaccines related to their durability, which appears to be only 2-4 months. This requires ongoing boosting in an attempt to maintain a higher level of antibody immunity to thwart the potential risk of severe disease infection. In addition, the current vaccines require an extreme frozen state, which means that we really can’t deliver and administer the vaccines the way we’d like in various regions of the world.
A major need for improvement relates to the inadequacy of the currently authorized vaccines to address the highest-at-risk patients – those with compromised immune systems. The existing Covid-19 vaccines are all focused on inducing a very strong antibody response towards reducing the risk of severe disease infection. Unfortunately, this is inadequate for millions of patients in the U.S. and worldwide. In the U.S., there are approximately 15 million people with medical conditions resulting in their bodies being depleted in the ability to adequately respond to antibody stimulation, including that from the current Covid-19 vaccines. There are over 240 million such patients estimated worldwide.
These immunocompromised patients have depleted immune systems as a result of having certain medical conditions — they may have blood cancers, be HIV positive, have sickle cell anemia, or have renal disease, or they may be on suppressant drugs that inhibit the body’s ability to respond to antibody stimulation due to having received organ or stem cell transplation. These are the patients who remain within the pandemic, seeking Covid-19 vaccines that provide adequate protective immunity against severe Covid-19 infection, hospitalization and potential death.
The GeoVax next-generation Covid-19 vaccine, currently in three Phase 2 human clinical trials, differs from the current authorized vaccines in that it induces a broad immune response, including not only an antibody response as does the current vaccines but, also a very strong T-cell response. In other words, our Covid-19 vaccine induces strong antibody response in both the antibody and cellular immune systems. This is the result of our novel vaccine construct which incorporates two critical components of the SARS-CoV-2 virus.
The authorized vaccines focus on an element of the spike protein to induce a strong antibody response. Their vaccine technology is unable to incorporate more than one antigen from the virus. Our vaccine technology differs in enabling multiple antigens to be incorporated into the vaccine.
The GeoVax COVID-19 vaccine achieves a strong T-cell response by including a particular antigen called the nucleocapsid protein, typically referred to as the “N protein”. The combination of the S and N proteins in our vaccine have demonstrated in human clinical trials to result in potent antibody and cellular immune responses. In fact, the clinical results have demonstrated that our vaccine provides potent protective immunity from the initial Wuhan strain and through delta and omicron/XBB1.5. We’re currently testing the latest variant, JN.1.
Thus far, the clinical results support that our vaccine, in contrast to the current authorized vaccines, maintains protective immune response levels without the need for reconfiguration.
It is critically important that Covid-19 vaccines provide durability lasting at least more than 6 months. The clinical results of our vaccine continue to demonstrate durability of six-to-eight months, representing approximately twice or more of what is now recognized from the authorized vaccines. In two of our three Phase 2 clinical trials, we are conducting direct comparisons against the mRNA vaccines. We believe that there is a potential to demonstrate that our Covid-19 vaccine is more robust (broader immune responses) and more durable (longer-lasting) than the mRNA vaccines.
Himanshu: So, this COVID vaccine is developed using MVA and existing vaccines i.e. Pfizer vaccine and others are mRNA vaccines. So can you tell us what is the difference between both the technologies and also eventually the vaccines?
David: There are major differences between MVA as the vaccine platform used in GEO-CM04S1 and the platforms mRNA platform used by Pfizer/BioNTech, Moderna, as well as the adenovirus platform used in the Oxford/Astra-Zeneca vaccine. First, mRNA and adenovirus vaccine platforms do nothing until an antigen of interest (e.g., the SARS-Cov-2 “Spike” antigen) is inserted in order to induce a strong Antibody response in vaccinated patients. MVA is different in that it is actually a vaccine as described in the response to Q2. That means that (a) the MVA platform induces immune responses regardless of a target antigen being inserted – MVA is the authorized vaccine used to prevent Mpox (“Monkeypox”) and Smallpox; (b) MVA, differing from mRNA and adenovirus platforms, allows for the insertion of multiple antigens – in the case of our Covid-19 vaccine, the “Spike” protein and the “Neucleocapsid” protein, resulting in induction of both strong Antibody and strong Cellular (e.g., T-cells) immune responses. Clinical results indicate that this provides a more robust, durable immune response; (c) Covid-19 vaccines developed based upon the mRNA and adenovirus platform have needed to be updated/reconfigured as new variants have emerged. Clinical results for our Covid-19 vaccine have demonstrated protective immune responses from the ancestral Wuhan strain through Delta, Omicron and, the recent XBB.1.5, without the need for reconfiguration. Finally, (d) MVA-based vaccines do not require the extreme frozen state as do the mRNA or the extreme refrigeration of the adenovirus vaccines (“cold chain supply”), requiring either moderate refrigeration or even delivered in a freeze-dried/lyophilized state. Of course, since MVA is already well accepted by regulatory authorities worldwide, there are no potential “safety surprise” that may become evident as MVA-based vaccines are distributed to mass patient populations worldwide.
Himanshu: What is the clinical status of the vaccine trial? We could see you have chosen a very selected patient population what is the reason? Please tell us the reason for such as unique trial design.
David: Currently, there are three Phase 2 clinical trials underway with GEO-CM04S1:
-
Booster vaccine trial among Healthy Volunteers, initially receiving a mRNA vaccine
This trial is fully-enrolled with 63 patients, having completed enrolment last September. The study evaluates our vaccine as a universal booster provided to healthy adults who initially received a mRNA vaccine. The patients are followed and evaluated for a year, meaning that final results will be available at the beginning of Q4 this year. On 2/6/24, we issued a press release announcing the positive, promising results from the initial, interim evaluation. We also noted that the vaccine has provided potential protective immunity against emerging variants without the need to reconfigure or update our vaccine – this is a critically important unique benefit we’re observing
-
Primary vaccine trial among immunocompromised/stem cell transplant patients
This trial is a direct, randomized comparison of our vaccine vs mRNA vaccines, evaluating a primary vaccine among immunocompromised/stem cell transplant patients. This population is recognized as a highest-risk for severe infection, hospitalization and potential death. As published in the New England Journal of Medicine (D. Barouch; 8.31.2022), antibodies are necessary for addressing the initial Covid-19 infection but, T-cells are critically important to reducing the risk of severe infection, hospitalization and potential death. These patients have had their immune systems ablated in preparation for stem cell transplantation, eliminating their ability to respond adequately to antibody stimulation. Our vaccine, also inducing strong cellular/T-cell immunity, is anticipated to provide a more robust immune response, as well as more durable response, providing improved protective immunity to this high-risk patient population.
Initial clinical data were published in Vaccines (9/15/2023), demonstrating the strong immune responses – both antibody and cellular – resulting from our vaccine vs mRNA. The publication also noted that our vaccine has demonstrated protective immunity from the Wuhan strain through Omicron XBB.1.5.
In addition to the initial site at City of Hope National Medical Center, there are now four additional sites (U.Mass; Fred Hutchinson Cancer Center; Wake Forest; Eastern Carolina Medical Center) actively enrolling patients. Additional results are anticipated to be reported yet this year.
-
Booster vaccine trial among immunocompromised/Chronic Lymphocytic Leukemia (CLL) patients
CLL represent another high-risk, immunocompromised patient population for which the current authorized vaccines are inadequate as a result of their bodies being depleted in the ability to appropriately respond to antibody stimulation. This trial is an investigator-initiated-trial (ITT), conducted at City of Hope (two locations: Durate and Orange County, CA), direct, randomized study comparing our vaccine vs the Pfizer/BioNTech mRNA vaccine as a booster to CLL patients, having initially received a mRNA vaccine. Interim results are anticipated during the first Half, 2024. This study is being funded by a private family foundation but, GeoVax has rights to the data in support of our overall development of GEO-CM04S1.
Our focus for GEO-CM04S1 is to develop the vaccine as the primary Covid-19 vaccine for those with compromised immune systems. As previously noted, there are 15 million such, high-risk individuals in the U.S. and over 240 million worldwide. We believe that expedited regulatory pathway exists for the development of our vaccine focused on such patients, especially as a result of the highly recognized inadequacy of the currently authorized vaccines among immunocompromised patient populations.
GeoVax holds worldwide rights to GEO-CM04S1 and expects worldwide development, registration and commercialization in conjunction with partners and collaborations. Such discussions are currently underway with encouraging interest.
Himanshu: Let's us about the clinical status, trial design, results status of the SCCHN study?
David: Gedeptin recently completed a Phase 1/2 clinical trial as therapy against advanced head and neck cancer. This was a FDA-supported trial, primarily to document safety, but also evaluating anti-tumor effects. The results, as reported at an international conference last July, demonstrate safety and consistent reduction in treated tumors. Those results are currently under final review, as well as our plans for an expanded Phase 2 trial, of approximately 40-60 patients, which we believe may provide an expedited registration path. In the U.S., there are annually 15,000 individuals who die from advanced head and neck cancer. These patients have failed on all forms of therapy and are on end-stage palliative care. Our goal with this indication is to provide some level of improved quality of life, via treated tumors, perhaps improving their ability to swallow or speak. This also represents a priority within FDA, which is why they have supported the current trial.
We’re currently discussing with oncology experts regarding an expanded Phase 2 trial as a potential basis for registration. In addition, we’re discussion which additional tumors to address with Gedeptin. This also includes combination therapy of Gedeptin and an immune checkpoint inhibitor, potentially enhancing the therapeutic performance versus either therapy administered separately. We’ve been supporting animal studies of such combination therapy with the results being highly encouraging. We look forward to proceeding with the next stage of Gedeptin development, which we believe will provide multiple, novel options to address early and late-stage tumors.
GeoVax holds worldwide rights to the Gedeptin technology for all indications and we anticipate developing this widely worldwide, in conjunction with partners and collaborators. We are in active and encouraging such discussions.
Himanshu: Can we talk about the other programs of GeoVax?
David: Following Gedeptin and GEO-CM04S1, our next development priority is GEO-MVA, which represents the development, registration and commercialization of MVA as a stand-alone vaccine. As previously noted, MVA is authorized as the vaccine to prevent Smallpox and Mpox (“Monkeypox”). Globally, there currently is a single supplier, which is unable to supply global demand and needs. GeoVax expects to change this – sooner than later. We acquired from the U.S. NIH the rights to develop, manufacture and commercialize MVA as a stand-alone vaccine against Mpox and Smallpox. We believe that there is an expedited path to deveop and register GEO-MVA, eliminating the current global monopoly and, most important, providing sufficient supply to meet global demands and need. Along with the transition to the advanced MVA manufacturing process, as discussed in the response to Q2, we anticipate eliminating the current requirement to stockpile MVA, producing product in real-time to meet whatever needs might exist.
Many people may ask why we need vaccines for diseases that we don’t have here in the United States. Well, we closed down the world for coronavirus, which had a 1.5–2.5% fatality rate.
Meanwhile, at the very top of the list in biodefense or potential bioterrorism are hemorrhagic fever viruses —the Ebola family, meaning Ebola Zaire and Ebola Sudan and the Marburg virus — these types of viruses have a 50–90% percent fatality rate. We need vaccines for these not because we think there might be an outbreak necessarily in the U.S., but because the U.S. government and other free societies recognize that such viruses represent the greatest potential bioterrorism threats. In other words, “weaponized viruses”.
To help prepare for this type of situation, we have developed different vaccines. The NIH and the U.S. military have supported our developments in these areas. To date, we have completed non-human primate studies for vaccines against Ebola zaire, Ebola sudan and Marburg.
Previously, we showed 100% protection in a single dose through non-human primates with Ebola Zaire. More recently, our vaccine demonstrated approximately 80% protection with two doses against Ebola Sudan, and similar results with Marburg. We’re in discussions with companies who have biodefense as part of their core business regarding continued development with the goal of distribution of these vaccines into the U.S. Strategic National Stockpile. While our focus remains on the areas of therapies against cancer and our vaccine against Covid-19, we anticipate that our biodefense developments will advance through such partnerships, addressing critically important needs against potential bioterrorism threats.
Himanshu: Which of these programs is expected to enter clinics first?
David: That will depend on partners and/or government agencies. We don’t anticipate leading the further development of these vaccines but, we recognize the high value that they potentially provide relative to endemic areas throughout the world and for biodefense.
Himanshu: Lastly, before we finish Can we talk a little about you as well for our audience (your background, experience, you vision for GeoVax)?
David: During his career, Mr. Dodd has overseen the approval of over 10 NDAs, over 15 acquisitions/divestitures, in excess of $2.5 billion in financial transactions, and over $5 billion in incremental enterprise growth. Mr. Dodd’s achievements also include successful IPO listings, re-capitalizations, and corporate developments in multiple international jurisdictions.
David Dodd has served as the President and CEO of GeoVax Labs, Inc. since 2018. Mr. Dodd joined the GeoVax Board of Directors in March 2010 and was elected Chairman in January 2011. Since being appointed President, and CEO, he has led GeoVax through a corporate recapitalization and up-listing to Nasdaq, followed by the Company acquiring the exclusive worldwide rights to two Phase 2 status products. This includes GEO-CM04S1, a next-generation vaccine for Covid-19, currently in multiple Phase 2, multi-site clinical trials, and Gedeptin®, a cancer immunotherapy having recently completed enrollment of a multi-site Phase 1/2 trial, funded by the FDA Orphan Drugs Clinical Trials Program.
During Mr. Dodd’s six-year tenure as President, CEO, and Director of Serologicals Corporation (Nasdaq: SERO) the market value increased from $85 million to $1.5 billion, during which Mr. Dodd brokered an all-cash sale to Millipore Corporation. While serving as President, CEO & Director of Solvay Pharmaceuticals, Inc., the enterprise value increased over five years from $100 million to $2.5 billion. Mr. Dodd also served in executive roles with Wyeth-Ayerst Laboratories, now part of Pfizer, Bristol-Myers Squibb, and Abbott Laboratories.
Mr. Dodd holds Bachelor of Science and Master of Science degrees from Georgia State University and completed the Advanced Management Program of Harvard Business School.
Himanshu: Anything you would like to add or cover which you think is important about the company or product? (Here you can mention if we missed anything or if you are looking for partners or anything?)
David: GeoVax – strong IP; holding worldwide rights for all of our assets/developments; focused on partnering/collaborations to ensure worldwide development, regisration and commercialization.
Himanshu: Bharat Biotech’s Covaxin is also developed using attenuated virus and your vaccine is developed using similar principle. Is there any difference between them and what would that be and how does that difference makes your vaccine better than Bharat biotech as they are similar?
David: I believe that Covaxin is killed, not attenuated. It will induce antibodies and helper T-cells (CD4) but not killer T-cells (CD8). The dominant antigens will be S and N. Our GEO-CM04S1 will be a better inducer of T-cells and the immunity should be more durable (based on past experience with MVA). Covaxin is similar to the Valneva product which looked promising early on but has failed as a booster.
Image Source: Canva
About the Speaker:
.png)
David A. Dodd
Chairman, President & CEO
David Dodd is a recognized leader with highly successful experience and expertise in transforming and restructuring public and private companies, establishing strong governance processes and operational discipline, resulting in operational improvements, significant revenue and enterprise value growth. During his career, he has overseen the approval of over 10 NDAs, over 15 acquisitions/divestitures, more than $2.5B in financial transactions and led over $5B in incremental enterprise growth.
Mr. Dodd joined the GeoVax Board of Directors in March 2010 and was elected Chairman in January 2011. Effective September 2018, he began serving also as the President, CEO. Mr. Dodd has led GeoVax through a significant transformation, including a corporate recapitalization and up-listing to Nasdaq (September 2020), followed by the Company acquiring the exclusive worldwide rights to two Phase 2-status products. This included GEO-CM04S1, the most advanced next-generation vaccine for Covid-19 (currently in two Phase 2 trials) and Gedeptin®, a cancer immunotherapy in a multi-site Phase 2 trial, having received Orphan Drug designation from the FDA and currently funded by the FDA Orphan Drugs Clinical Trials Program. In addition, significant strengthening of corporate governance, Board development and organizational capabilities have been implemented. Key highlights of his career prior to the GeoVax CEO position include:
During Mr. Dodd's six-year tenure as President, CEO and Director of Serologicals Corporation (Nasdaq “SERO”), the market value of that company increased over $1B… from $85M when he joined SERO to an all-cash sale to Millipore Corporation for $1.5B;
Dodd served as President, CEO & Director of Solvay Pharmaceuticals, Inc., during which the enterprise value increased over $2B – from $100M to $2.5B over a five-year period;
He is a frequent invited speaker and panelist at business and economic development conferences, as well as various conferences focused on the life sciences and pharmaceutical industry. Mr. Dodd is a multi-year recipient of the FastTech 50 Growth Company Award while leading the transformation of Serologicals Corporation and, in 2022, he was selected as a recipient of the Georgia Titan 100 award.
In addition, he has served on a development committee of the MacArthur Foundation, as an advisor to the First Minister of Scotland, as the Chair of the American Foundation for Suicide Prevention, as a member of the External Advisory Board of the Petit Institute for Bioengineering & Biosciences (GaTech), as the Chair of GaBio, as a board member on the Harvard Business School Healthcare Alumni Association and, in leadership roles with numerous additional organizations. Earlier in his career (1988), Mr. Dodd was recognized by the Los Angeles City Council for his leadership in establishing a developmental childcare facility with Womens’ Hospital in the East L.A. area.
Mr. Dodd holds Bachelor of Science and Master of Science degrees from Georgia State University and completed the Harvard Business School of Advanced Management Program.
Related Post: Spotlight Interview: David A. Dodd, CEO at GeoVax Labs, in an Engaging Conversation with PharmaShots
Tags

Himanshu brings over 12 years of Pharma, Life Sciences, and Biotech expertise to Pharmashots. A self-proclaimed "Pharma Geek," his passion for the industry shines through. His career path blends hands-on lab research with high-level consulting at firms like PwC-Health Industries and Novartis. Himanshu holds a degree in Immunology from the University of Leeds, and boasts further credentials from IIM Lucknow and the London School of Economics. Connect with Himanshu to dive deeper into the dynamic world of pharmaceuticals and life sciences.













